Principal Investigator
Prof. Dr. Lars Redecke & Prof. Dr. Thomas Schulz
& Prof. Dr. Melanie Brinkmann
Universität zu Lübeck
& Hannover Medical School
& Technische Universität Braunschweig
C1
PhD candidate
Fatama Sornaly



C1
Project Summary
Structural and functional features of a conserved DNA-binding domain in herpes viral proteins as a basis for innovative targeted therapies

Human herpesviruses establish lifelong persistent infections that can play a key role in tumorigenesis and provide the basis for severe disease resulting from viral reactivation. Herpesviral proteins interacting with viral DNA (vDBPs) are key players in latency establishment, maintenance, and regulation. In the herpesviral replication cycle, vDBPs can be classified as latent or immediate-early (IE) lytic proteins. Latent proteins such as KSHV LANA-1 and EBV EBNA-1 of the gamma herpesvirus subfamily mediate the replication of latent viral episomes and their partitioning to daughter cells during mitosis. On the other hand, lytic proteins such as HHV-6A IE2 and HCMV IE2 of the beta herpesvirus subfamily act as transcriptional activators of early or late viral promoters.
Based on the crystal structures, the DNA-binding domains (DBDs) of LANA-1, EBNA-1, and HHV-6A IE2 share significant structural homology. AlphaFold models of HCMV IE2 predict a similar fold structure which indicates that DBD is conserved among herpesvirus families. In LANA-1, there are two caspase I and III cleavage sites. We hypothesize that a physiologically relevant domain is formed in the C-terminal domain (CTD) after being cleaved by caspase III, which we refer to as the extended CTD (eCTD).
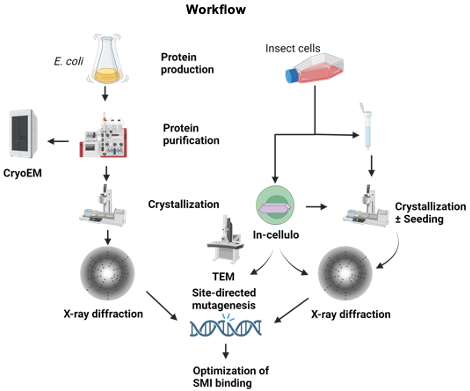
The aim of the project is the structural and functional characterization of LANA DBD, eCTD, and HCMV-IE2 CTD alone and in complex with the viral DNA and small molecular inhibitors (SMIs) to develop innovative targeted therapy. We plan to employ conventional X-ray crystallography, in cellulo crystallization techniques, and cryo-EM for structure elucidation
C1
References
- Schulz TF, Freise A, Stein SC. Kaposi sarcoma-associated herpesvirus latency-associated nuclear antigen: more than a key mediator of viral persistence. Curr Opin Virol. 2023 Aug;61:101336. doi: 10.1016/j.coviro.2023.101336.
- Nishimura M, Wang J, Wakata A, et al. Crystal Structure of the DNA-Binding Domain of Human Herpesvirus 6A Immediate Early Protein 2. J Virol. 2017 Oct 13;91(21):e01121-17. doi: 10.1128/JVI.01121-17.
- Hellert J, Weidner-Glunde M, Krausze J, et al. The 3D structure of Kaposi sarcoma herpesvirus LANA C-terminal domain bound to DNA. Proc Natl Acad Sci U S A. 2015 May 26;112(21):6694-9. doi: 10.1073/pnas.1421804112.
- Bochkarev A, Barwell JA, Pfuetzner RA, et al Crystal structure of the DNA-binding domain of the Epstein-Barr virus origin-binding protein EBNA 1. Cell. 1995 Oct 6;83(1):39-46. doi: 10.1016/0092-8674(95)90232-5.
- Berwanger A, Stein SC, Kany AM, et al Disrupting Kaposi’s Sarcoma-Associated Herpesvirus (KSHV) Latent Replication with a Small Molecule Inhibitor. J Med Chem. 2023 Aug 10;66(15):10782-10790. doi: 10.1021/acs.jmedchem.3c00990.
- Kirsch P, Jakob V, Oberhausen K, et al Fragment-Based Discovery of a Qualified Hit Targeting the Latency-Associated Nuclear Antigen of the Oncogenic Kaposi’s Sarcoma-Associated Herpesvirus/Human Herpesvirus 8. J Med Chem. 2019 Apr 25;62(8):3924-3939. doi: 10.1021/acs.jmedchem.8b01827.
- Kirsch P, Stein SC, Berwanger A, et al. Hit-to-lead optimization of a latency-associated nuclear antigen inhibitor against Kaposi’s sarcoma-associated herpesvirus infections. Eur J Med Chem. 2020 Sep 15;202:112525. doi: 10.1016/j.ejmech.2020.112525.
- Messick TE, Smith GR, Soldan SS, et al. Structure-based design of small-molecule inhibitors of EBNA1 DNA binding blocks Epstein-Barr virus latent infection and tumor growth. Sci Transl Med. 2019 Mar 6;11(482):eaau5612. doi: 10.1126/scitranslmed.aau5612.
- Redecke L, Nass K, DePonte DP, et al. Natively inhibited Trypanosoma brucei cathepsin B structure determined by using an X-ray laser. Science. 2013 Jan 11;339(6116):227-230. doi: 10.1126/science.1229663.
- Nass K, Redecke L, Perbandt M, et al. In cellulo crystallization of Trypanosoma brucei IMP dehydrogenase enables the identification of genuine co-factors. Nat Commun. 2020 Jan 30;11(1):620. doi: 10.1038/s41467-020-14484-w.
