Principal Investigator
Prof. Dr. Christian Hübner
Universität zu Lübeck
A05
PhD candidate
Leon Westermann

A05
Project Summary
Dimerisation of viral capsid proteins investigated by single molecule FRET
Human noroviruses (HuNoV’s) cause a large number of deaths every year [1]. As HuNoV’s are difficult to study, the murine norovirus (MNV) and the rabbit hemorrhagic disease virus (RHDV) are used as model systems to get a better understanding of the viral lifecycle [2]. Here, the P-domain of MNV and RHDVb, a new, less lethal but more infectious, variant of RHDV [3], are studied. The P-domain is part of the structural protein which builds up the viral capsid and is located on the outer part of the capsid [4]. For MNV it has been shown that the P-domain interacts with monoclonal antibodies [5], a cellular receptor, metal ions, and a bile acid [6]. The presence of the bile acid glycochenodeoxycholic acid (GCDCA) enhances the infectivity of the virus [6] and prevents binding of monoclonal antibodies [7, 8]. GCDCA and metal ions (e.g. Mg2+) promoting dimerisation of isolated P-domain monomers in solution [8, 9] and cryo-EM studies revealed a contraction of the whole capsid [7, 10]. For P-domains of RHDV and RHDVb the binding to histo-blood group antigens (HGBAs) has been known [11, 12]. Recently, the binding of bile acids and metal ions to the P-domain of RHDVb was shown [13].
Here the influence of different ligands on the MNV and RHDVb P-domain dimerisation is studied using single molecule FRET experiments and other biophysical methods (e.g. fluorescence lifetime measure- ments).
A05
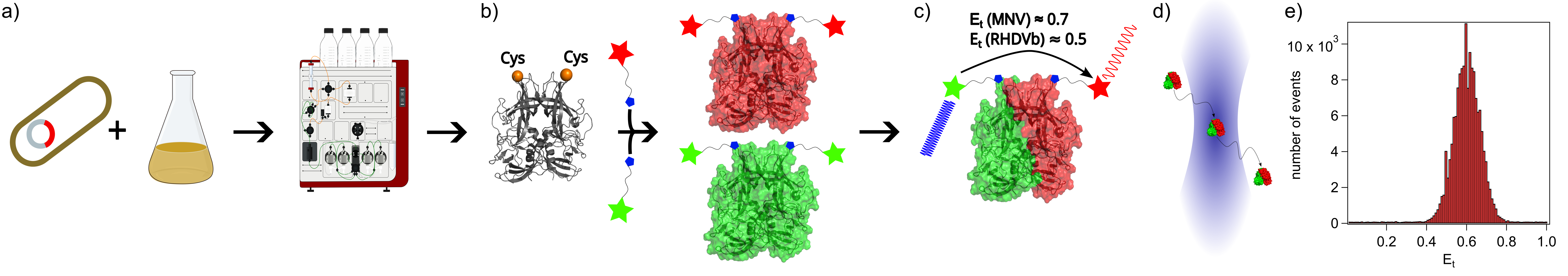
Experimental overview. (a) P-domains where expressed in E.coli and purified by affinity and size exclu- sion chromatography. (b) The proteins contain a cystein residue, inserted by site-directed mutagenesis, which can be labeled with a maleimid-fluorophor (Alexa Fluor 488/647) yielding an acceptor (red star) and a donor (green star) labeled protein species, respectively. (c) Monomer exchange may lead to the formation of hetero-labeled P-domain dimers which results in FRET-based acceptor photon emission upon donor excitation. (d) For the measurements a confocal microscope and pM protein concentrations are used, enabling excitation of single molecules which diffuse through the laser focus (blue). (e) The emitted fluorescent light is detected and the FRET efficiency (Et) can be calculated and depicted in a histogram. Crystal structures shown are from MNV (PDB 6E47).
A05
References
- Pires, S. M. et al. PLOS ONE 10, e0142927 (Dec. 3, 2015). https://doi.org/10.1371/journal.pone.0142927
- Vashist, S., Bailey, D., Putics, A. & Goodfellow, I. Future Virology 4, 353–367 (July 2, 2009). https://doi.org/10.1128/mbio.00215-20
- Le Gall-Recul ́e, G. et al. Veterinary Record 168, 137–138 (Feb. 2011). https://doi.org/10.1136/vr.d697
- Katpally, U. et al. Journal of Virology 84, 5836–5841 (June 2010). https://doi.org/10.1128/jvi.00314-10
- Katpally, U., Wobus, C. E., Dryden, K., Virgin, H. W. & Smith, T. J. Journal of Virology 82, 2079–2088 (Mar. 2008). https://doi.org/10.1128/jvi.02200-07
- Nelson, C. A. et al. Proceedings of the National Academy of Sciences 115 (Sept. 25, 2018). https://doi.org/10.1073/pnas.1805797115
- Williams, A. N. et al. Journal of Virology 95, e00176–21 (June 10, 2021). https://doi.org/10.1128/jvi.00176-21
- Creutznacher, R. et al. Communications Biology 5, 563 (June 9, 2022). https://doi.org/10.1038/s42003-022-03497-4
- Maaß, T. Dissertation, Universität zu Lübeck (2022).
- Sherman, M. B. et al. Journal of Virology 93 (2019). https://doi.org/10.1128/jvi.00970-19
- Leuthold, M. M., Dalton, K. P. & Hansman, G. S. Journal of Virology 89, 2378–2387 (Feb. 15,2015). https://doi.org/10.1128/jvi.02832-14
- Ruvoën-Clouet, N., Ganière, J. P., André-Fontaine, G., Blanchard, D. & Le Pendu, J. Journal of Virology 74, 11950–11954 (Dec. 15, 2000). https://doi.org/10.1128/jvi.74.24.11950-11954.2000
- König, P. Dissertation, Universität zu Lübeck (2023).
